Meningococcal B Vaccine Immunogenicity in Children With Defects in Complement and Splenic Function
Meningococcal B Vaccine Immunogenicity in Children With Defects in Complement and Splenic Function
Meningococcal B Vaccine Immunogenicity in Children With Defects in Complement and Splenic Function
Federico Martinón-Torres, PhD,a Ewa Bernatowska, MD,b Anna Shcherbina, MD,c Susanna Esposito, MD,d Leszek Szenborn, MD,e Magda Campins Marti, PhD,f Stephen Hughes, PhD,g Saul N. Faust, FRCPCH,h Luis I. Gonzalez-Granado, MD,i Ly-Mee Yu, PhD,j Diego D’Agostino, MSc,k Marco Calabresi, PhD,l Daniela Toneatto, MD,l Matthew D. Snape, MDm,n
abstract
BACKGROUND: The capsular group B meningococcal vaccine (4CMenB) is recommended for children with complement deficiencies, asplenia, and splenic dysfunction; however, data on the immunogenicity of 4CMenB in these “at-risk” children are missing.
METHODS: Participants aged 2 to 17 years in Italy, Spain, Poland, the United Kingdom, and Russia with complement deficiencies, asplenia, or splenic dysfunction received 2 doses of 4CMenB 2 months apart, as did healthy children in the control group. Exogenous and endogenous human complement serum bactericidal activity (SBA) was determined at baseline and 1 month after the second immunization against 4 test strains: H44/76 (assessing vaccine antigen factor H binding protein), 5/99 (Neisserial adhesion A), NZ98/254 (Porin A), and M10713 (Neisserial heparin binding antigen).
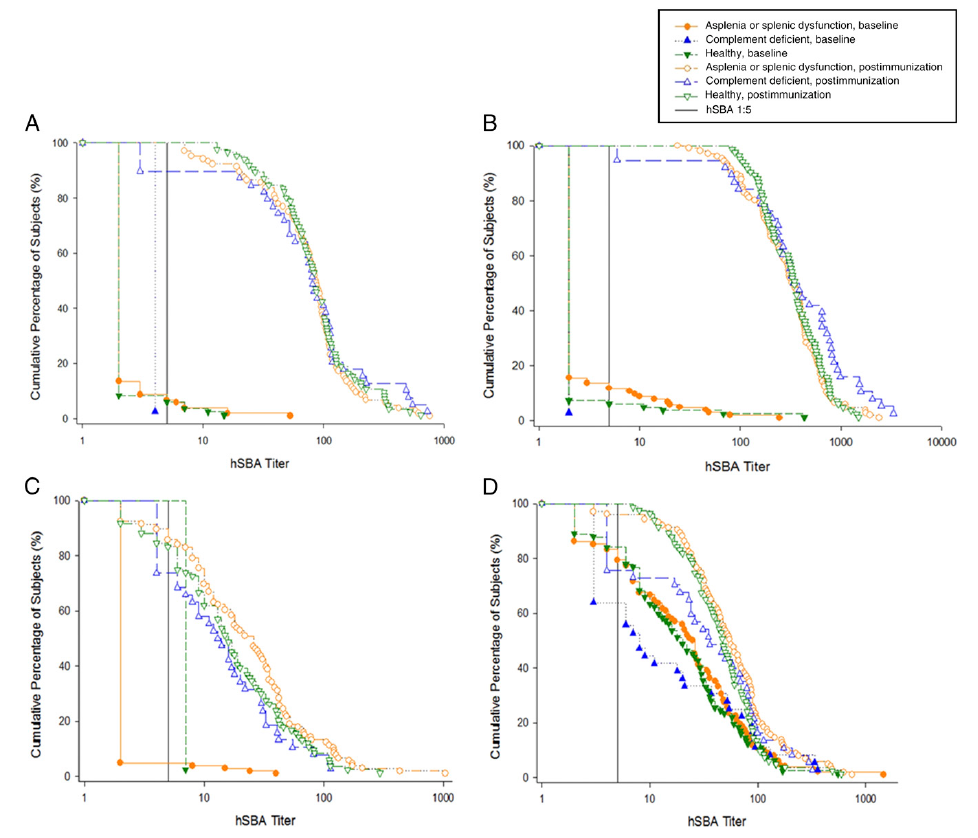
RESULTS: Of 239 participants (mean age 10.3 years, 45% female), 40 children were complement deficient (9 eculizumab therapy, 4 terminal-chain deficiencies, 27 “other”), 112 children had asplenia or splenic dysfunction (8 congenital asplenia, 8 functional asplenia, 96 splenectomy), and 87 children were in the control group. After immunization, the proportions of complement-deficient participants with exogenous complement SBA titers ≥1:5 were 87% (H44/76), 95% (5/99), 68% (NZ98/254), and 73% (M10713), compared with 97%, 100%, 86%, and 94%, respectively, for asplenic children and 98%, 99%, 83%, and 99% for children in the control group. When testing with endogenous complement, strain-specific bactericidal activity was evident in only 1 eculizumab-treated participant and 1 terminal chain complement-deficient participant.
CONCLUSIONS: 4CMenB administration is similarly immunogenic in healthy children and those with asplenia or splenic dysfunction. The significance of the trend to lower responses of SBA titers in complement-deficient children (especially those with terminal chain complement deficiency or those on eculizumab therapy) must be determined by ongoing surveillance for vaccine failures.
aTranslational Pediatrics and Infectious Diseases, Hospital Clinico Universitario de Santiago de Compostela, Santiago de Compostela, Spain; bDepartment of Immunology, The Children’s Memorial Health Institute, Warsaw, Poland; cResearch and Clinical Centre of Pediatric Hematology, Oncology and Immunology named after Dmitry Rogachev, Moscow, Russian Federation; dPediatric Clinic, Department of Surgical and Biomedical Sciences, Università degli Studi di Perugia, Perugia, Italy; eDepartment of Pediatric Infectious Diseases, Wroclaw Medical University, Wroclaw, Poland; fHospital Universitario Vall d’Hebron, Barcelona, Spain; gRoyal Manchester Children’s Hospital, Manchester, United Kingdom; hNational Institute for Health Research Wellcome Trust Clinical Research Facility, University of Southampton and University Hospital Southampton NHS Foundation Trust, Southampton, United Kingdom;iImmunodeficiencies Unit, Department of Pediatrics, University Hospital 12 de Octubre, Research Institute Hospital 12 Octubre (i+12) and Associate Professor of Pediatrics, Complutense
ARTICLE
WHAT’S KNOWN ON THIS SUBJECT: Group B meningococcal vaccine (4CMenB) immunization of infants prevents group B meningococcal disease. No data are available on the immunogenicity of 4CMenB in children with complement deficiency, who are at increased risk of disease, nor in children with splenic dysfunction.
WHAT THIS STUDY ADDS: In this study, we show that most children with complement deficiencies develop an immune response after immunization with 4CMenB, although this is reduced in children with terminal-chain complement deficiencies and on eculizumab therapy. Ongoing surveillance for vaccine failures is required.
| To cite: Martinón-Torres F, Bernatowska E, Shcherbina A, et al. Meningococcal B Vaccine Immunogenicity in Children With Defects in Complement and Splenic Function. Pediatrics. 2018;141(3):e20174250 |
The licensure of the capsular group B meningococcal vaccine (4CMenB) in Europe, Australia, and the Americas offers the potential for vaccine prevention of invasive disease because of this bacterium. This vaccine is now recommended for use in healthy infants in the United Kingdom, Ireland, and some regions of Germany, Italy, Canada. In addition, these and many other countries recommend (and reimburse) the use of 4CMenB in children, teenagers, and adults at increased risk of invasive meningococcal disease (IMD); in the United States, this recommendation applies only to preteenagers, teenagers, and adults. Such “at-risk” groups include those with terminal chain complement deficiencies known to have rates of IMD 7000 to 10 000 higher than the general population and those receiving the monoclonal antibody eculizumab, which acts by binding complement 5. In view of reports of overwhelming sepsis and elevated mortality, children with asplenia or splenic dysfunction are also included in these recommendations, although the incidence of IMD in these populations may not be higher than their healthy peers.
Although authors of previous studies have shown immunization of complement-deficient patients with polysaccharide capsular group A, C, W, and Y meningococcal vaccines to be effective against invasive disease, the use of 4CMenB in these populations has not previously been studied. Accordingly, in this study, we evaluated the immunogenicity and tolerability of 4CMenB in children with congenital or acquired complement deficiencies and with asplenia or splenic dysfunction and compared this with healthy children in the control group.
METHODS
This was an open-label phase IIIb study conducted at 18 sites (4 in Italy, 4 in Spain, 3 in Poland, 4 in the United Kingdom, and 3 in Russia) between May 2014 and March 2015.
Researchers in the study recruited children aged 2 to 17 years into 1 of the following 3 categories: “complement deficient, ” “asplenia/splenic dysfunction” or “healthy controls.” Children in the complement deficient category were those diagnosed by their clinician as having a primary deficiency (a congenital deficiency leading to a reduced concentration of 1 or more proteins in the complement cascade, including C1, C2, C3, C4, factor D, properdin, C5, C6, C7, C8, C9, factor H, or homozygous factor I) or a secondary deficiency (conditions indirectly leading to a reduced concentration of 1 or more proteins in the complement cascade, including those treated with eculizumab). Children in the asplenia or splenic dysfunction category were those with congenital anomalies of the spleen, surgical splenectomy, or autosplenectomy (eg, in patients with sickle cell disease). Healthy controls were healthy age-matched children. Children were excluded if they had previously received any meningococcal group B vaccine or if they had suffered IMD within the last year (complement deficient or asplenia or splenic dysfunction groups) or at any time previously (healthy controls). Participants could not be on any antibiotics within 3 days before enrollment other than those prescribed for prophylaxis, and immunization was deferred until at least 6 hours after the administration of analgesics or antipyretics. Full inclusion and exclusion criteria are presented in the Supplemental Information.
The study intervention was 2 doses of 4CMenB (Bexsero, GSK, Italy) administered intramuscularly 2 months apart. As previously described, 19 each 0.5 mL dose of vaccine contains 50 μg each of purified factor H binding protein (fHBP), Neisserial heparin binding antigen (NHBA), Neisserial adhesion A antigens, and 25 μg of outer membrane vesicle component with immunodominant porin protein (Porin A).
The primary immunogenicity objective was to evaluate the immunogenicity of 2 doses of 4CMenB in participants with increased risk of meningococcal disease because of complement deficiency or asplenia and in healthy age-matched children at 1 month after the second vaccination. Immunogenicity for this primary objective was determined by using exogenous human complement assays measuring serum bactericidal activity (hSBA) against 4 test strains: H44/76 (assessing vaccine antigen fHBP), 5/99 (Neisserial adhesion A), NZ98/254 (Porin A), and M10713 (NHBA). hSBA assays were performed at the Clinical Sciences Laboratory, Novartis Vaccines (now GlaxoSmithKline Biologicals SA), Marburg. Exogenous human complement was added to serial diluted sera after the addition of bacteria solution. For assays with complement-independent bactericidal activity, suggestive of antibiotic killing, the hSBA assays were repeated in the presence of β-lactamase. For hSBA and β-lactamase–hSBA assays, the threshold of response was 1:5, as previously reported.
In an additional exploratory analysis, serum bactericidal assays were also performed by using endogenous complement alone (serum bactericidal activity using endogenous complement [endhSBA]). For these assays, dilution was only performed to a 1:8 titer, in view of the inherent limitations of complement dilution in the absence of exogenous complement, and the threshold of response was 1:4.
The primary safety objective was to assess the safety and tolerability of 2 doses of 4CMenB in these children. During the week after a vaccination, parents recorded the local injection site and systemic symptoms. Separate classification systems were used for children aged 2 to 5 years old and 6 to 17 years old, as shown in Supplemental Figs 4 and 5. Adverse event recording was enhanced by telephone contact during the week after study vaccination. All serious adverse events reported during the study were recorded.
The primary population for immunogenicity analysis was the full analysis set, consisting of all participants who received a study vaccination and provided postimmunization serum that was evaluable for at least 1 of the study strains. The primary immunogenicity end points were, for each of the strains at baseline and 1 month after the second dose of vaccine, the percentages of participants with hSBA titers ≥1:5, hSBA ≥1:8, and with a fourfold rise in hSBA titers above baseline, along with 95% Clopper confidence intervals (CIs). Between-group comparisons were performed through computation of the 2-sided 95% CI for the differences in percentages between the study groups. For end-hSBA titers, the percentage of participants with titers ≥1:4 and hSBA ≥1:8 was calculated.
The hSBA geometric mean titers (GMTs) and associated 2-sided 95% Clopper-Pearson CIs were constructed by exponentiating the least square means of the corresponding log-transformed hSBA titers and their 95% CIs obtained from an analysis of variance with study group (complement deficient and asplenia or splenic dysfunction) and study site as model factors. For the healthy controls group, raw unadjusted GMTs and 95% CIs were calculated by exponentiating the means of the logarithmically transformed titers and their 95% CIs. Geometric mean ratios of hSBA titers post- and preimmunization were calculated, and ratios of postimmunization hSBA GMTs between groups and their 95% CIs were calculated for comparisons of immune responses between the at-risk groups and healthy controls group. Titers below the limit of detection were set to half that limit for the purpose of analysis. The percentage of participants experiencing vaccine reactions were analyzed for each dose separately, according to the age groups 2 to 5 years and 6 to 17 years.
The planned sample size was ∼240 participants, with ∼160 in the at-risk groups (with at least 15 from each of the complement-deficient and the asplenia or splenic dysfunction groups) and ∼80 children in the control category. Recruitment of the healthy controls group occurred in a 1:2 ratio for at-risk children, stratified according to site and to the age groups 2 to 5 years, 6 to 10 years, and 11 to 17 years. A sample size of 80 participants in a group was calculated to provide a 95% CI of 81.2% to 95.6% around an observed percentage “response” of 90.0%.
TABLE 1 Participant Demographics
| Complement Deficient | Asplenia or Splenic Dysfunction | Healthy | Total | |
| n = 40 | n = 112 | n = 87 | n = 239 | |
| Age in y, mean ± SD | 8.5 ± 4.4 | 11.1 ± 3.7 | 10.2 ± 4.1 | 10.3 ± 4.1 |
| Sex, n (%) | ||||
| Male | 23 (58) | 66 (59) | 43 (49) | 132 (55) |
| Female | 17 (43) | 46 (41) | 44 (51) | 107 (45) |
| Country of enrollment, n (%) | ||||
| Italy | 7 (18) | 14 (13) | 12 (14) | 33 (14) |
| Poland | 8 (20) | 29 (26) | 20 (23) | 57 (24) |
| Russia | 4 (10) | 24 (21) | 17 (20) | 45 (19) |
| Spain | 13 (33) | 29 (26) | 24 (28) | 66 (28) |
| United Kingdom | 8 (20) | 16 (14) | 14 (16) | 38 (16) |
| Race, n (%) | ||||
| Asian | 1 (3) | 0 | 1 (1) | 2 (1) |
| Black or African American | 0 | 4 (4) | 1 (1) | 5 (2) |
| White | 36 (90) | 105 (94) | 84 (97) | 225 (94) |
| Other | 3 (8) | 3 (3) | 1 (1) | 7 (3) |
RESULTS
Across 18 sites, 239 participants were enrolled (mean age 10·35 years, 45% female), 40 of whom were complement deficient (9 eculizumab therapy, 4 terminalchain deficiencies, 1 with factor H deficiency, 4 with factor I deficiency, 2 with C1 deficiency, 5 with C2 deficiency, 14 with C3 and/or C4 deficiency, and 1 with alternative pathway deficiency). One hundred and twelve participants had asplenia or splenic dysfunction (8 congenital asplenia, 8 functional asplenia, and 96 splenectomy), and 87 were participants in the control category. Further details of participant demographics, their underlying diagnoses, and the study flowchart are shown in Table 1, Fig 1, and Supplemental Table 3.

Immunogenicity
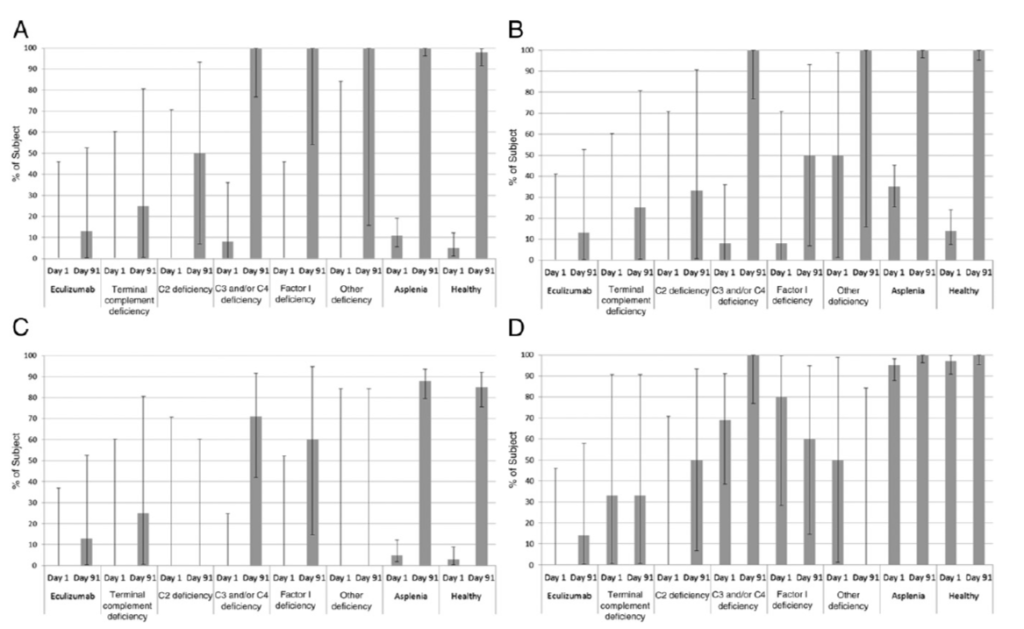
When tested with serum bactericidal activities (SBAs) by using exogenous human complement, no complementdeficient participants had baseline hSBA titers ≥1:5 for strains H44/76, 5/99, and NZ98/254 (and no more than 12% of participants with asplenia and 6% of participants in the control category). Baseline seropositivity rates for M10713 were 56% (participants who were complement deficient), 79% (participants with asplenia), and 78% (participants in the control category). After immunization, the proportions of complement-deficient participants with hSBA titers ≥1:5 (by using exogenous complement) rose to 87% (H44/76), 95% (5/99), 68% (NZ98/254), and 73% (M10713), compared with 97%, 100%, 86%, and 94%, respectively, for the asplenia/splenic dysfunction group, and 98%, 99%, 83%, and 99% for participants in the control category (Table 2, Fig 2). Between-group comparisons revealed the point estimate of the percentage of “responders” was 4% to 26% lower in participants who were complement-deficient than in the healthy control group; but this was statistically significant for strains H44/76 and M10713 only. No such trend to a lower response was seen for participants in the asplenic/ splenic dysfunction group compared with the healthy control group. GMTs and percentage of participants with SBA titers ≥1:8 revealed a broadly similar pattern (Supplemental Tables 4 and 5). The analyses of the percentage of participants developing hSBA titers ≥1:5 and hSBA GMTs by complement deficiency type revealed that of 8 participants on eculizumab therapy with hSBA results, only 4 (H44/76), 6 (5/99), 2 (NZ98/254), and 1 (M10713) had hSBA titers ≥1:5 postimmunization (Supplemental Tables 6 and 7). By contrast, all 4 participants with terminal chain complement deficiencies achieved these thresholds for each strain.
Fewer than half of complementdeficient and asplenic/splenic dysfunction participants reported taking antibiotics during the study, and only 1% to 2.8% of participants per group had evidence of complement independent killing on hSBA assays that would be suggestive of antibiotic activity. The bactericidal assays on these participants were repeated by using pretreatment with β-lactamase (additional exploratory analyses), but given the low numbers, this had minimal impact on overall response rates or GMTs (data not shown).
TABLE 2 Percentage of Analyzed Subjects (and 95% CIs) With hSBA Titers ≥ 1:5
| Strain | Timing | Complement Deficient | Asplenia or Splenic Dysfunction | Healthy |
| n = 39 | n = 106 | n = 85 | ||
| H44/76 | Baseline, No. (%) (95% CI) | 0 (0%) (0.0% to 9.0%) | 7 (7%) (2.7% to 13.4%) n = 104 | 5 (6%) (2.0% to 13.3%) n= 84 |
| 1 mo after second vaccination, No. (%) (95% CI) | 34 (87%) (72.6% to 95.7%) | 101 (97%) (91.8% to 99.4%) n = 104 | 83 (98%) (91.8% to 99.71%) | |
| Study group differences 1 mo after second vaccination, % (95% CI) | Complement deficient to healthy −10% (−24.6% to −1.6%) | Asplenia or splenic dysfunction to healthy −1% (−6.1% to 5.6%) | ||
| Baseline, No. (%) (95% CI) | 0 (0%) (0.0% to 9.5%) n = 37 | 12 (12%) (6.2% to 19.5%) n = 103 | 5 (6%) (2.0% to 13.7%) n = 82 | |
| 5/99 | 1 mo after second vaccination, No. (%) (95% CI) | 36 (95%) (82.3% to 99.4%) n = 38 | 106 (100%) (96.6% to 100.0%) | 82 (99%) (93.5% to 99.97%) n = 83 |
| Study group differences 1 mo after second vaccination, % (95% CI) | Complement deficient to healthy −4% (−16.3% to 2.2%) | Asplenia or splenic dysfunction to healthy 1% (−2.3% to 6.5%) | ||
| NZ98/254 | Baseline, No. (%) (95% CI) | 0 (0%) (0.0% to 9.7%) n = 36 | 4 (4%) (1.0% to 9.5%) n = 105 | 2 (2%) (0.29% to 8.4%) n = 83 |
| 1 mo after second vaccination, No. (%) (95% CI) | 26 (68%) (51.3% to 82.5%) n = 38 | 91 (86%) (77.7% to 91.9%) | 70 (83%) (73.6% to 90.6%) n = 84 | |
| Study group differences 1 mo after second vaccination, % (95% CI) | Complement deficient to healthy −15% (−32.4% to 0.8%) | Asplenia or splenic dysfunction to healthy 3% (−7.8% to 13.5%) | ||
| M10713 | Baseline, No. (%) (95% CI) | 20 (56%) (38.1% to 72.1%) n = 36 | 81 (79%) (70.3% to 86.8%) n = 102 | 64 (78%) (67.5% to 86.4%) n = 82 |
| 1 mo after second vaccination, No. (%) (95% CI) | 27 (73%) (55.9% to 86.2%) n = 37 | 97 (94%) (87.8% to 97.8%) n = 103 | 82 (99%) (93.5% to 99.97%) n = 83 | |
| Study group differences 1 mo after second vaccination, % (95% CI) | Complement deficient to healthy −26% (−42.0% to −13.7%) | Asplenia or splenic dysfunction to healthy −5% (−11.1% to 1.3%) |
When using endogenous complement, postimmunization bactericidal activity at 1:4 dilution was observed in 68% (H44/76), 60% (5/99), 41% (NZ98/254), and 60% (M10713) of complement-deficient participants, compared with 98%, 100%, 85% and 100%, respectively, in the healthy control group (Supplemental Table 8). These percentages varied according to diagnosis, with only 1 of the 4 terminal chain complement deficient participants (reported as having a C7 deficiency) and only 1 of the 8 eculizumab recipients displaying any bactericidal activity postimmunization (Fig 3, Supplemental Table 9). For children with asplenia, when using endogenous complement, bactericidal activity at 1:4 dilution was seen in 11% (H44/76), 35% (5/99), 5% (NZ98/254), and 95% (M10713), rising to 100%, 100%, 88%, and 100%, respectively, postimmunization.
Reactogenicity
Fever rates per immunization among 2- to 5-year-olds were 17% in complement-deficient children after each dose, 11% after each dose in children with asplenia, and 31% (first dose) and 8% (second dose) for children in the control category. Among 6- to 17-year-olds, these rates were 18% after the first dose and 4% after the second dose in complementdeficient participants; per dose fever rates were 3% to 4% for all other participants. Full details of solicited systemic and local adverse events are shown in Supplemental Figs 4 and 5. There were 7 serious adverse events in 6 participants (appendicitis, gastroenteritis, 2 respiratory infections, concussion, tonsillitis, and intracardiac thrombus), none of which were considered related to immunization.
DISCUSSION
In this study, we provide the first data to inform existing recommendations for use of 4CMenB in children at increased risk of meningococcal disease. After 2 doses of the 4CMenB vaccine, children with asplenia or splenic dysfunction had bactericidal activity against 4 test strains that was similar to children in the control category. Immunization with 4CMenB was also able to generate bactericidal activity in the presence of exogenous complement in the majority of children with complement deficiency, although this response was lower when tested by using endogenous complement and among those with terminal chain complement deficiencies or on eculizumab treatment.
The central role for complement in in vivo killing of meningococci (by generation of the terminal membrane complex) is reflected in the use of the serum bactericidal assay as the accepted correlate of protection against IMD in clinical trials of meningococcal vaccines.16 Rather than only testing the quantity of antigen-specific antibodies induced by immunization, SBA assays provide a qualitative test of the ability of these antibodies to kill meningococci in the presence of complement. Assessing the immunogenicity of meningococcal vaccines in children with acquired or congenital complement deficiencies is therefore problematic because testing the immune response in these children with exogenous complement from “healthy” donors could overestimate the protection that immunization is likely to afford.
While bearing this important caveat in mind, an increase in bactericidal activity was observed in most children with non–terminal chain complement deficiencies, whether measured with exogenous or endogenous complement. By contrast, and not unexpectedly, children on eculizumab had poor responses even when tested with exogenous complement, presumably secondary to binding of the exogenous complement. Similarly, it is surprising that even a single participant with a terminal chain complement deficiency or undergoing treatment with eculizumab demonstrated an increase in bactericidal activity when tested with endogenous complement.
Of relevance to this study is the experience with the use in immunocompromised patients of other meningococcal vaccines based on capsular antigens. The best direct evidence of the ability for antibodies against capsular antigens to provide protection, even in the absence of functional (endogenous) complement, comes from an observational study of 45 patients with terminal chain complement deficiency, all of whom were offered the plain polysaccharide capsular A, C, W, and Y meningococcal vaccine. Of the 31 patients receiving the vaccine, 6 episodes of IMD were observed over a 2-year observation period, compared with 6 out of 14 who did not receive the vaccine; the mean time to first episode of IMD was ∼8 years in vaccine recipients compared with ∼5 in nonrecipients, a difference that was statistically significant. Although complement deficient individuals have an impaired antibody response to polysaccharide based vaccines, sera from these individuals did display a postimmunization increase in killing of serogroup A and W meningococci that was dependent on the presence of polymorphonuclear leukocytes, suggesting that some protection was afforded by opsonophagocytosis rather than complement mediated killing. Although these data are based on immunity against capsular polysaccharides rather than subcapsular proteins, Plested et al provide encouraging evidence that sera from adults immunized with a precursor to 4CMenB is able to kill meningococci ex vivo (in both passive protection and opsonophagocytic assays), even in the absence of complement-dependent bactericidal activity. This again suggests a role for opsonophagocytosis in protecting patients with primary complement deficiency against IMD, an aspect of the immune response that was not evaluated in this study. Of note is that this additional mode of killing may not apply to patients treated with eculizumab; in recent data, it is suggested that opsonophagocyticdependent killing of meningococci in a whole blood assay is blocked in the presence of eculizumab.
Reassuringly, children with asplenia or splenic dysfunction generate an immune response that is at least as good as children in the control category. This contrasts with the reduced immunogenicity of the serogroup C meningococcal protein–polysaccharide vaccines in adults with surgical asplenia reported by Balmer et al. These contrasting findings may reflect the older age group in the later study or potential differences in the immune response of patients with asplenia to capsular polysaccharides rather than subcapsular proteins. Given this robust immune response to 4CMenB, it is reasonable to expect that this vaccine will be as effective in children with asplenia or splenic deficiency as in children in the control category. It is also reassuring, although not unexpected, that the reactogenicity profile in the at-risk children was broadly similar to children in the control category and to previously reported studies in these age groups.
Ultimately, the best understanding of the role of 4CMenB in immunocompromised patients will come from surveillance for vaccine failures. To date, there are 2 published case reports of group B IMD in eculizumab-treated patients who had received a full course of 4CMenB and 1 fatal case of IMD due to an unencapsulated strain bearing fHBP and NHBA proteins similar to those in 4CMenB. Together with reports of group C, W, and Y IMD in such patients after immunization with MenACWY vaccines, these cases may be used to support the above concerns regarding the ability of immunization to generate protective immunity against IMD in the presence of eculizumab.
More formal calculations of vaccine effectiveness will include a comparison of rates of disease in immunized versus nonimmunized at risk individuals on eculizumab and with other at-risk conditions, and comparing these to the vaccine failure rate observed in age-matched healthy children. Ongoing surveillance is crucial, and will also inform whether specific schedules for at-risk vaccine recipients may be required, eg, with additional booster doses. Given the incomplete coverage of 4CMenB for all capsular group B strains and the uncertainty regarding the effectiveness of the 4CMenB vaccine in the most at-risk populations, it should be emphasized that immunization with 4CMenB does not remove the need for ongoing antibiotic prophylaxis in those for whom it is currently recommended. The low rates of antibiotic use in this study and lack of evidence of antibiotic activity in the serum of the vast majority of study participants does raise the possibility that adherence to this recommendation is poor.
This study had a number of limitations, among them the relatively small number of children with terminal chain complement deficiencies and on eculizumab therapy. Additionally, the diagnosis of complement deficiency was based on local clinical diagnosis, with no formal standardized diagnostic criteria (eg, evidence of previous invasive pneumococcal or miningococcal infection) or central laboratory testing of complement function. This potentially allowed inclusion of participants with partial complement deficiencies, which may be of particular relevance to participants with reported C4 deficiencies (for which partial deficiency is much more common than total deficiency), and to the participant with reported C7 deficiency who developed bactericidal activity postimmunization. Also, in this study, we did not include those with HIV infection, known to be at increased risk of disease, nor did we address the immunogenicity of the licensed vaccine schedules for immunocompromised children <2 years of age. Finally, as previously described, many participants had SBA titers ≥5 preimmunization for strain M10713 (evaluating the NHBA component of 4CMenB). Nevertheless, the increase in GMTs observed does provide some support for the immunogenicity of this vaccine antigen in the populations studied.
CONCLUSIONS
In the data from this study, provisional support for the existing guidelines for immunization against capsular group B disease in children with complement deficiencies and splenic dysfunction is provided. Ongoing surveillance for vaccine failures is required to determine the significance of the trend to reduced immune response in children with terminal chain complement deficiencies or undergoing treatment with eculizumab. In the meantime, it is important that these patients are identified, receive education about sepsis management plans, and are prescribed prophylactic antibiotics according to local guidelines, along with vaccination, to provide every chance for them to be protected against this deadly disease.
ACKNOWLEDGMENTS
We thank Richard Sewell, Hannah Robinson, Tim Sell, Austen Worth, Paolo Durando, Caterina Cancrini, Fernando Baquero Artigao, Annaelisa Tasciotti, Vasundhara Dindore, Gema Ariceta, Maurizio De Martino, Jose Sánchez de Toledo, Sonia Maria Uriona, and JoAnne Welsch for their contributions to the study. We acknowledge the support of Tiberia Pop for the preparation of the immunogenicity and reactogenicity figures and Maria Ana de la Grandiere for manuscript coordination (both XPE Pharma and Science on behalf of GSK).
ABBREVIATIONS
4CMenB: capsular group B meningococcal vaccine
CI: confidence interval
end-hSBA: serum bactericidal activity using endogenous complement
fHBP: factor H binding protein
GMT: geometric mean titer
hSBA: human complement assays measuring serum bactericidal activity
IMD: invasive meningococcal disease
NHBA: Neisserial heparin binding antigen
SBA: serum bactericidal activity
University of Madrid, Madrid, Spain; jNuffield Department of Primary Care Health Sciences and mOxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, UnitedKingdom; kGlaxoSmithKline, Amsterdam, The Netherlands; lGlaxoSmithKline, Siena, Italy; and nNational Institute for Health Research Oxford Biomedical Research Centre, Oxford, United Kingdom
Drs Snape and Martinón-Torres contributed to the acquisition of data, analysis of the data, and prepared the first draft of the manuscript; Drs Toneatto and Calabresi contributed to the study conception, design, and interpretation of the data; Dr Esposito, Dr Szenborn, Dr Bernatowska, Dr Shcherbina, Dr Marti, Prof Faust, Dr Hughes, and Prof Gonzalez-Granado conducted the study and contributed to the acquisition of data and the interpretation of the data; Mr D’Agostino and Dr Yu provided statistical expertise and contributed to the analysis and interpretation of the data; and all authors had full access to all the data in the study, take responsibility for the integrity of the data and the accuracy of the data analysis, reviewed and commented critically on drafts of the manuscript for important intellectual content, and approved the final manuscript as submitted.
Mr D’Agostino’s current affiliation is Document IT BeNeLux B.V., Amsterdam, The Netherlands.
Dr Calabresi’s current affiliation is Covance Inc, Laboratory Corporation of America Holdings (LabCorp), Rome, Italy.
This trial has been registered at www. clinicaltrials. gov (identifier NCT02141516).
DOI: https:// doi. org/ 10. 1542/ peds. 2017- 4250
Accepted for publication Mar 8, 2018
Address correspondence to Matthew D. Snape, MD, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford OX3 7LE, United Kingdom. E-mail: matthew.snape@paediatrics.ox.ac.uk
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2018 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by Novartis Vaccines and Diagnostics (now GlaxoSmithKline Biologicals SA), who designed the study protocol and conducted the initial analysis of the data.
POTENTIAL CONFLICT OF INTEREST: The institution of Dr Martinón-Torres received clinical trial fees from Novartis during the conduct of this study, and he received personal fees and/or nonfinancial support and/or grants and/or other from Novartis, Pfizer, Sanofi-Pasteur MSD (SPMSD), the GlaxoSmithKline (GSK) group of companies, and/or Merck outside the submitted work. Prof Esposito reports grants from Novartis, the GSK group of companies, Pfizer, Sanofi-Pasteur, and Valeas, and personal fees from Novartis, the GSK group of companies, Pfizer, Sanofi-Pasteur, Novavax, and Vifor outside the submitted work. Prof Szenborn received personal fees from Novartis and the GSK group of companies during the conduct of the study. The institution of Prof Marti received clinical trial fees from Novartis during the conduct of the study, and she received grants from Novartis, GSK, and Pfizer outside of the submitted work. The institution of Prof Faust received grants from GSK group of companies during the conduct of the study and received grants for his participation in advisory boards (Astra Zeneca and Cubist) and speaking engagements (Pfizer). Prof Faust acts as an investigator for clinical studies from both noncommercial funding bodies and commercial sponsors (ie, some or all of Novartis Vaccines, the GSK group of companies, MedImmune and/or AstraZeneca, and Pfizer Vaccines) conducted on behalf of Southampton University Hospital NHS Foundation Trust and the University of Southampton. The institution of Prof Gonzalez-Granado received clinical trial fees from Novartis during the conduct of the study and he has received grants for his participation in advisory boards and speaking engagements (CSL Behring and Baxter). Dr Calabresi and Mr D’Agostino were employees and Dr Toneatto is an employee of the GSK group of companies. Dr Toneatto owns stock and/or stock options in the GSK group of companies. Dr Snape acts as an investigator for clinical studies from both noncommercial funding bodies and commercial sponsors (ie, some or all of Novartis Vaccines, the GSK group of companies, Sanofi-Aventis, SPMSD, MedImmune, and Pfizer Vaccines) conducted on behalf of the University of Oxford and Oxford University Hospitals NHS trust. The NIHR Oxford Biomedical Research Centre provides salary support for Dr Snape, who is a Jenner Investigator; the other authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www. pediatrics. org/ cgi/ doi/ 10. 1542/ peds. 2018- 0554.
REFERENCES
1. Committee for Medicinal Products for Human Use. European Medicines Agency. Summary of Product Characteristics. Bexsero. Available at: http:// www. ema. europa. eu/ docs/ en_ GB/ document_ library/ EPAR_-_ Product_ Information/ human/ 002333/ WC500137881. pdf. Accessed April 4, 2018 2. Australian Register of Therapeutic Goods. ARTG ID 190719 - BEXSERO. 2013. Available at: http:// search. tga. gov. au/ s/ search. html? collection= tga- artg& profile= record& meta_ i= 190719. Accessed June 25, 2016 3. US Food and Drug Administration. BEXSERO. 2015. Available at: https:// www. fda. gov/ BiologicsBloodVac cines/ Vaccines/ApprovedProducts/ ucm431374.htm.Accessed June 25, 2016 4. Bianchi A, Fantoni S, Prugnola A. Meningococcal B vaccine and the vision of a meningitis free world. J Prev Med Hyg. 2015;56(3):E140–E143 5. Canadian Paediatric Society. Update on invasive meningococcal vaccination for Canadian children and youth. Available at: https:// www. cps. ca/ en/ documents/ position/invasivemeningococcalvaccination. Accessed April 4, 2018 6. JCVI. JCVI position statement on use of Bexsero meningococcal vaccine in the UK. 2014. Available at: https:// www. gov. uk/ government/ publications/ meningococcal- b- vaccine- jcvi- positionstatement. Accessed June 25, 2016 7. National Immunisation Advisory Committee. Immunisation guidelines for Ireland chapter 13 Meningococcal infection. 2016. Available at: www.hse. ie/ eng/ health/ immunisation/ hcpinfo/ guidelines/ chapter13. pdf. Accessed June 25, 2016 8. Dubé E, Gagnon D, Hamel D, et al. Parents’ and adolescents’ willingness to be vaccinated against serogroup B meningococcal disease during a mass vaccination in Saguenay-Lac-St-Jean (Quebec). Can J Infect Dis Med Microbiol. 2015;26(3):163–167 9. Signorelli C, Chiesa V, Odone A. Meningococcal serogroup B vaccine in Italy: state-of-art, organizational aspects and perspectives. J Prev Med Hyg. 2015;56(3):E125–E132 10. Public Health England. Immunisation against infectious disesase. Chapter 7, immunisation of individuals with underlying medical conditions. 2014. Available at: www. gov. uk/ government/ uploads/ system/ uploads/ attachment_ data/ file/ 309218/ Green_ Book_ Chapter_ 7_ v1_ 3. pdf. Accessed June 25, 2016 11. Hellenbrand W, Koch J, Harder T, et al. Background paper for the update of meningococcal vaccination recommendations in Germany: use of the serogroup B vaccine in persons at increased risk for meningococcal disease. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58(11–12):1314–1343 12. Pinto MV, Bihari S, Snape MD. Immunisation of the immunocompromised child. J Infect. 2016;72(suppl):S13–S22 13. Center for Disease Control and Prevention. Meningococcal vaccination: what everyone should know. 2017. Available at: https:// www. cdc. gov/ vaccines/ vpd/ mening/ public/ index. html. Accessed April 4, 2018 14. Figueroa J, Andreoni J, Densen P. Complement deficiency states and meningococcal disease. Immunol Res. 1993;12(3):295–311 15. Hillmen P, Muus P, Röth A, et al. Longterm safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013;162(1):62–73 16. Holdsworth RJ, Irving AD, Cuschieri A. Postsplenectomy sepsis and its mortality rate: actual versus perceived risks. Br J Surg. 1991;78(9):1031–1038 17. Holmes FF, Weyandt T, Glazier J, Cuppage FE, Moral LA, Lindsey NJ. Fulminant Meningococcemia after splenectomy. JAMA. 1981;246(10):1119 1120 18. Platonov AE, Vershinina IV, Kuijper EJ, Borrow R, Käyhty H. Long term effects of vaccination of patients deficient in a late complement component with a tetravalent meningococcal polysaccharide vaccine. Vaccine. 2003;21(27–30):4437–4447
19. Gossger N, Snape MD, Yu LM, et al; European MenB Vaccine Study Group. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA. 2012;307(6):573–582 20. Biselli R, Casapollo I, D’Amelio R, Salvato S, Matricardi PM, Brai M. Antibody response to meningococcal polysaccharides A and C in patients with complement defects. Scand J Immunol. 1993;37(6):644–650 21. Platonov AE, Vershinina IV, Käyhty H, Fijen CA, Würzner R, Kuijper EJ. Antibody-dependent killing of meningococci by human neutrophils in serum of late complement componentdeficient patients. Int Arch Allergy Immunol. 2003;130(4):314–321 22. Plested JS, Welsch JA, Granoff DM. Ex vivo model of meningococcal bacteremia using human blood for measuring vaccine-induced serum passive protective activity. Clin Vaccine Immunol. 2009;16(6):785–791 23. Konar M, Granoff DM. Eculizumab treatment and impaired opsonophagocytic killing of meningococci by whole blood from immunized adults. Blood. 2017;130(7):891–899 24. Balmer P, Falconer M, McDonald P, et al. Immune response to meningococcal serogroup C conjugate vaccine in asplenic individuals. Infect Immun. 2004;72(1):332–337 25. McQuaid F, Snape MD, John TM, et al. Persistence of bactericidal antibodies to 5 years of age after immunization with serogroup B meningococcal vaccines at 6, 8, 12 and 40 months of age. Pediatr Infect Dis J. 2014;33(7):760–766 26. McQuaid F, Snape MD, John TM, et al. Persistence of specific bactericidal antibodies at 5 years of age after vaccination against serogroup B meningococcus in infancy and at 40 months. CMAJ. 2015;187(7):E215–E223 27. Santolaya ME, O’Ryan ML, Valenzuela MT, et al; V72P10 Meningococcal B Adolescent Vaccine Study group. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet. 2012;379(9816):617–624 28. Hernando Real S, Vega Castaño S, Pajares García R. Meningococcemia in vaccinated patient under treatment with eculizumab. Enferm Infecc Microbiol Clin. 2017;35(3):200–201 29. Parikh SR, Lucidarme J, Bingham C, et al. Meningococcal B vaccine failure with a penicillin-resistant strain in a young adult on long-term Eculizumab. Pediatrics. 2017;140(3):e20162452 30. McNamara LA, Topaz N, Wang X, Hariri S, Fox L, MacNeil JR. High risk for invasive Meningococcal disease among patients receiving Eculizumab (Soliris) despite receipt of Meningococcal vaccine. Am J Transplant. 2017;17(9):2481–2484 31. Cullinan N, Gorman KM, Riordan M, Waldron M, Goodship TH, Awan A. Case report: benefits and challenges of long-term eculizumab in atypical hemolytic uremic syndrome. Pediatrics. 2015;135(6). Available at: www.pediatrics. org/ cgi/ content/ full/135/ 6/ e1506 32. Struijk GH, Bouts AH, Rijkers GT, Kuin EA, ten Berge IJ, Bemelman FJ. Meningococcal sepsis complicating eculizumab treatment despite prior vaccination. Am J Transplant. 2013;13(3):819–820 33. Grumach AS, Kirschfink M. Are complement deficiencies really rare? Overview on prevalence, clinical importance and modern diagnostic approach. Mol Immunol. 2014;61(2):110 117 34. Miller L, Arakaki L, Ramautar A, et al. Elevated risk for invasive meningococcal disease among persons with HIV. Ann Intern Med. 2014;160(1):30–37 35. Simmons RD, Kirwan P, Beebeejaun K, et al. Risk of invasive meningococcal disease in children and adults with HIV in England: a population-based cohort study. BMC Med. 2015;13:297 36. Snape MD, Voysey M, Finn A, et al; European MenB Vaccine Study Group. Persistence of bactericidal antibodies after infant serogroup B Meningococcal immunization and booster dose response at 12, 18 or 24 months of age. Pediatr Infect Dis J. 2016;35(4):e113–e123
