A Conditioning Regimen with Plerixafor Is Safe and Improves the Outcome of TCRαβ+ and CD19+ Cell-Depleted Stem Cell Transplantation in Patients with Wiskott-Aldrich Syndrome
A Conditioning Regimen with Plerixafor Is Safe and Improves the Outcome of TCRαβ+ and CD19+ Cell-Depleted Stem Cell Transplantation in Patients with Wiskott-Aldrich Syndrome
Biol Blood Marrow Transplant 24 (2018) 1432–1440


Biology of Blood and
Marrow Transplantation
journal homepage: www.bbmt.org
Dmitry Balashov1, Alexandra Laberko2, Anna Shcherbina2, Pavel Trakhtman3, Dmitrii Abramov4, Elena Gutovskaya1, Svetlana Kozlovskaya1, Larisa Shelikhova1, Galina Novichkova5, Michael Maschan1, Alexander Rumiantsev5, Alexei Maschan1 1 Department of Hematopoietic Stem Cell Transplantation, Dmitriy Rogachev National Center for Pediatric Hematology, Oncology, and Immunology, Moscow, Russia 2 Department of Immunology, Dmitriy Rogachev National Center for Pediatric Hematology, Oncology, and Immunology, Moscow, Russia 3 Transfusion Medicine Service, Dmitriy Rogachev National Center for Pediatric Hematology, Oncology, and Immunology, Moscow, Russia 4 Department of Pathology, Dmitriy Rogachev National Center for Pediatric Hematology, Oncology, and Immunology, Moscow, Russia 5 Medical Department, Dmitriy Rogachev National Center for Pediatric Hematology, Oncology, and Immunology, Moscow, Russia
Article history:
Received 16 January 2018
Accepted 7 March 2018
_________________________
Key Words:
Hematopoietic stem cell
transplantation TCRαβ+ cell depletion Wiskott-Aldrich syndrome Plerixafor Conditioning regimen Chimerism
A B S T R A C T
Our initial experience with hematopoietic stem cell transplantation (HSCT) from a matched unrelated donor (MUD; n = 12) or a haploidentical related donor (n = 6) with T cell receptor (TCR)αβ+/CD19+ graft depletion in patients with Wiskott-Aldrich syndrome (WAS) (n = 18) showed a dramatic decrease in the incidence of graft-versus-host disease (GVHD) and transplantation-related mortality, with an increased overall survival (OS) of 88.9%. Unfortunately, the treatment was associated with mixed myeloid donor chimerism and secondary graft dysfunction (severe thrombocytopenia, n = 2; graft rejection, n = 5). To improve the outcome, we hypothesized that the addition of G-CSF and plerixafor to the conditioning chemotherapy would result in more complete donor stem cell engraftment. This trial was registered at www.clinicaltrials.gov (NCT03019809). A study group of patients withWAS (n = 16) underwent TCRαβ+/CD19+-depleted HSCT (MUD, n = 6; haploidentical, n = 10). The conditioning regimen was treosulfan-fludarabine-rabbit antithymocyte globulin-melphalan (or thiophosphamide in 1 patient) with G-CSF (10 μg/kg/day for 5 days starting on day −8) and plerixafor (240 μg/kg/day for 3 days starting on day −6). The clinical outcomes in this study were compared to those in a historical dataset (n = 18). No patients had grade III/IV acute GVHD in either the study or the historical control group. Importantly, in the patients with WAS, there was no statistical significance in OS between those who underwent HSCT from haploidentical donors and those who underwent HSCT from MUDs (93.8% versus 88.5%; P = .612). All patients in the study group had full donor chimerism in whole blood and in the CD3+ compartments. The OS was 93.8%, and there were no cases of graft dysfunction. This study demonstrates the efficacy of adding G-CSF/plerixafor to the conditioning regimen before HSCT with TCRαβ+/C D19+ graft depletion in patients with WAS.
© 2018 American Society for Blood and Marrow Transplantation.
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) is curative for many primary immunodeficiency (PID) disorders. Most patients have no HLA-identical family donor, and thus technologies have evolved to provide safe and effective HSCT from unrelated or related haploidentical donors. T cell receptor (TCR)αβ+/CD19+ depletion is a modern method of graft engineering that has proven effective in preventing graft-versushost disease (GVHD) and providing satisfactory immune recovery kinetics [1-6]. However, the experience of TCRαβ+/CD19+ graft depletion is limited to several studies in heterogeneous groups of patients, and available data for specific disorders are scarce [3,4].
Wiskott-Aldrich syndrome (WAS) is one of the most frequently diagnosed PID conditions. Patients with the classic WAS phenotype are highly predisposed to bacterial, viral, and opportunistic infections [7]. Furthermore, 24% to 72% of patients develop autoimmune or inflammatory complications and are at high risk for developing malignancies (mostly Epstein-Barr virus-associated lymphomas and leukemias) [8-11]. Therefore, HSCT has become the gold standard ofWAS treatment. HSCT with TCRαβ+/CD19+ depletion has been successfully used in our center since 2012 [12]. It has dramatically decreased the incidence of GVHD, and transplantationrelated mortality (TRM) and has improved overall survival (OS) in patients with PID, including those withWAS. However, the approach has been associated with a number of WAS cases with secondary graft dysfunction and mixed myeloid chimerism. A high incidence of graft dysfunction is one of the most common problems after HSCT in patients with WAS. Moratto et al. [13] found that 27.9% of 194 patients withWAS who underwent HSCT had mixed chimerism, more frequently in the myeloid compartment (16.5%) compared with the T lymphoid compartment (3.2%) or B lymphoid compartment (7.4%). Mixed myeloid chimerism often leads to graft rejection or persistence of severe thrombocytopenia [13,14]. Autoimmune complications are frequently associated with mixed myeloid chimerism [13,15].
Presumably, mixed myeloid chimerism in WAS can be caused by the low susceptibility of early progenitors to chemotherapy and the protective effects of the bone marrow microenvironment [16]. The approach of including plerixafor and G-CSF administration in the pre-HSCT conditioning regimen has been described in several reports [17-19]. The effect is based on mobilizing bone marrow stemcells into the peripheral blood and blocking CXCR4 chemokine receptors to prevent stem cell homing. Thus, some have hypothesized that plerixafor and G-CSF make free stromal space of the bone marrow available for donor stem cell engraftment. Konopleva et al. [18] found a low incidence of mixed chimerism after plerixafor/G CSF administration in addition to a nonmyeloablative conditioning regimen in patients with acute myelogenous leukemia. Dvorak et al. [19] reported their experience with the use of G-CSF and plerixafor alone as a preparative regimen for patients with severe combined immunodeficiency (SCID) undergoing HSCT; however, the efficacy of this approach was not described, possibly because of the high proliferative potential of stem cells without chemomyeloablation, which leads to stem cell homing to free bone marrow niches.
In an effort to improve the outcome of HSCT in patients withWAS, we initiated a prospective trial to evaluate the efficacy of adding G-CSF and plerixafor to the preparative regimen. This trial has been registered at www.clinicaltrials.gov as a prospective, interventional, single-group, openlabel study (NCT03019809). The results of the trial were compared to findings in a historical dataset.
MATERIALS AND METHODS
Between October 2012 and July 2017, 32 patients with WAS underwent 34 allogeneic HSCTs in our center (Table 1). The median age at diagnosis was .5 years (range, .06 to 11.7 years). The diagnosis of WAS was based on the European Society for Immunodeficiencies criteria [20] and confirmed by the detection of WAS gene mutations in all cases. Of the 32 patients, 20 had a severity score of 4 or 5 based on the Ochs scoring system [14]. Twelve patients had thrombocytopenia with mild immunodeficiency (1 to 3 on the Ochs scoring system). The median age at transplantation was 2 years (range, 0.7 to 12.6 years). Eighteen patients underwent HSCT from MUDs (10/10 HLA-matched, n 13; 9/10 HLA-matched, n = 4; 8/10 HLA matched, n = 1), and 16 underwent HSCT from parental haploidentical donors. In all cases, TCRαβ+/CD19+ graft depletion was used with the immune-magnetic method (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Two of 31 patients (patients 27 and 32 in Table 1) underwent 2 HSCTs with TCRαβ+/CD19+ graft depletion, and 1 patient (patient 21 in Table 1) underwent HSCT with TCRαβ+/CD19+ graft depletion after failure of the first HSCT, performed with a graft from an MUD with post-transplantation cyclophosphamide (days +3 and +4). Information on patients 1 to 8 has been published previously [12].
Patients with WAS who underwent HSCT between October 2012 and May 2016 served as the historical control group. All patients withWAS who underwent HSCT with TCRαβ+/CD19+ graft depletion between May 2016 and July 2017 were included in this prospective trial for evaluating the efficacy of adding G-CSF and plerixafor to the preparative regimen. All parents provided consent according to the European Group for Blood and Marrow Transplantation and local center regulations. The patients who received HSCT with G-CSF and plerixafor were specified as the study group.
Historical Control Group
Eighteen patients who had various chemotherapy conditioning regimens were included in the historical control group. Ten of these patients received conditioning with treosulfan (36 to 42 g/m2 total dose on days −5, −4, and −3) and fludarabine (150 mg/m2 total dose on days −6, −5, −4, −3, and −2). Six patients also received melphalan (140 mg/m2 on day −2), and 2 patients received cyclophosphamide (120 mg/kg) instead of melphalan. As serotherapy, 13 patients received rabbit anti-thymocyte globulin (rATG; 5 mg/kg on days −5 and −4) and 4 patients received horse antithymocyte globulin (90 mg/kg on days −6, −5, and −4). Fifteen of the 18 patients were given rituximab (100 mg/m2 on day −1) to decrease the risk of Epstein-Barr virus-related post-transplantation lymphoproliferative disease (EBV-PTLD).
In the historical group, 6 patients underwent HSCT from haploidentical donors and 12 underwent HSCT from MUDs (8 – 10/10 HLA-matched, n = 8; 9/10 HLA-matched, n = 3; 8/10 HLA-matched, n = 1). For GVHD prophylaxis, 9 patients received tacrolimus (T steady state, 8 to 12 ng/mL on days +1 to +60), and another 9 received tacrolimus combined with a short course of methotrexate (5 mg/m2 on days +1, +3, and +6).
Study Group
The study group comprised 16 patients (including 3 patients receiving a second HSCT). The conditioning regimen consisted of treosulfan 36 to 42 g/m2 total dose on days −5, −4, and −3; fludarabine 150 mg/m2 total dose on days −6, −5, −4, −3, and −2; and melphalan 140 mg/m2 on day −2 (with thiophosphamide 10 mg/kg on day −6 instead of melphalan in 1 patient), with the addition of G-CSF 10 μg/kg on days −8, −7, −6, −5, and −4 and plerixafor 240 μg/kg on days −6, −5, and −4. All patients received rATG 5 mg/kg on days −5 and −4 and rituximab 100 mg/m2 on day −1 (Figure 1).
Ten of 16 patients in the study group underwent HSCT from haploidentical donors, and 6 underwent HSCT from MUDs (10/10 HLAmatched, n = 5; 9/10-HLA matched, n = 1). For GVHD prophylaxis, all study group patients received tacrolimus from days +1 to +60.
Prevention and Treatment of Viral Infections
Cytomegalovirus (CMV), EBV, and adenovirus (ADV) viremia were monitored weekly in blood by quantitative PCR. Antiviral therapy with ganciclovir or foscarnet in neutropenic patients was initiated after any positive CMV PCR and was continued until 2 negative results were obtained. Regular ophthalmoscopy and intraocular injections of antiviral drugs (in addition to systemic antiviral therapy) was the optimized approach for the early detection and treatment of CMV retinitis.
Engraftment and Chimerism Analysis
Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count >.5 × 109/l, and platelet engraftment was defined as the first of 3 consecutive days with a platelet count >20.0 × 109/L unsupported by transfusion. Donor cell chimerism was assessed in whole blood and in the CD3+ lymphocyte compartment by quantitative PCR evaluation of differential short tandem repeat DNA sequences, as described previously [21]. Full chimerism was defined as the presence of >90% donor cells; mixed with predominantly donor chimerism, as a percentage of donor cells ranging from >50% to 90%; mixed with predominantly recipient chimerism, as a percentage of donor cells ranging from 10% to 50%; and null donor chimerism, as <10% donor cells. Null donor chimerism in whole blood was considered graft rejection.
Statistical Analysis
The data were analyzed in October 2017. The minimum follow-up period was 3 months. The patients were censored at the time of death or at the last follow-up. The baseline patient characteristics, transplant compositions, and donor types in the historical and study groups were compared using the Mann-Whitney U test and Fisher’s exact test. The probabilities of OS and event-free survival (EFS) were estimated by applying the Kaplan-Meier product limit method and were expressed as percentage ± standard error with 95% confidence interval (CI). The events considered for the EFS analysis were death, graft rejection, and thrombocytopenia (peripheral blood platelet level <100 × 109/L) after the first HSCT. Acute and chronic GVHD were diagnosed and graded according to standard guidelines [22]. The probabilities of acute and chronic GVHD, graft failure, and TRM were calculated as cumulative incidence curves ± standard error, with 95% CI to adjust the analysis for competing risks. The significance of the differences in chimerism levels was estimated by applying Fisher’s exact test in the contingency tables. Statistical analyses were conducted with XLSTAT 2015 software (Addinsoft, Paris, France). Boxplot graphs were created to display the time of engraftment, with median and first and third quartiles, 1.5 interquartile range whiskers, and 95% CI. Differences in the timing of engraftment were assessed with the Mann-Whitney U test using Rstudio software (RStudio, Boston, MA). The median with first and third quartiles was denoted as Me Q1 and Q3, respectively.
Table 1
Patient Characteristics, HSCT Features, and Outcomes
| Patient | Gene mutation | Donor type | Conditioning regimen | GVHD | Main clinical problems | Graft dysfunction | Outcome (days alive or death) | |
| Acute | Chrоnic | |||||||
| Historical control group | ||||||||
| 1 | Ex1;c.37C>T,p.R13Ter | MUD | Treo, Flu, h-ATG | IIgr(s,gi) | — | — | +1822 | |
| 2 | Ex 10; c.1031dupC, p.V345CfsTer150 | MUD | Treo, Flu, h-ATG | IIgr(s,gi) | — | — | +1695 | |
| 3 | Ex10;c.981_982dupCC, p.R328PfsTer118 | MUD | Treo, Flu, h-ATG | II gr (s) | — | — | +1646 | |
| 4 | Ex4;с.397G>A,p.E133K | Haplo | Treo, Flu, h-ATG | II gr (s) | — | — | +1547 | |
| 5 | IVS 6; c.559 + 5G>A | MUD | Treo, Flu, rATG | — | — | — | +1457 | |
| 6 | c.1221delG,p.N408MfsTer37 | MUD | Treo, Flu, rATG | — | — | Reject+70 | +1336 | |
| 7 | IVS 3; c.361-3C>G | MUD | Treo, Flu, rATG | — | — | CMV-ret | yes | +1178 |
| 8 | Ex4; с.397G>A, p.E133K | Haplo | Treo, Flu, rATG | — | — | CMV-ret | yes | +1155 |
| 9 | Ex1; с.71C>T, p.S24F | MUD | Treo, Flu, rATG | I gr (s) | — | CMV-ret | — | +1077 |
| 10 | Ex1; c.91G>A, p. E31K | MUD | Treo, Flu, rATG | — | — | ADV-pneum | — | Death+37 |
| 11 | Large deletion Ex1-12 | MUD | Treo, Flu, Cy | — | — | CMV- ret | Reject +82 | +1028 |
| 12 | Large deletion Ex12 | MUD | Treo, Flu, Mel, rATG | — | — | — | +932 | |
| 13 | Ex10;с.1271dupG, p.L425PfsTer70 | Haplo | Treo, Flu, Mel, rATG | — | — | — | +706 | |
| 14 | Large deletion Ex1-12 | Haplo | Treo, Flu, Mel, rATG | IIgr(s,gi) | — | — | +700 | |
| 15 | Large deletion Ex1-12 | Haplo | Treo, Flu, Cy, rATG | I gr (s) | — | CMV- ret | Reject +55 | +681 |
| 16 | Ex3;с.294delA,p.E98DfsTer29 | Haplo | Treo, Flu, Mel, rATG | — | — | Reject+207 | +545 | |
| 17 | Ex4; c.385G>C, p.A129P | MUD | Treo, Flu, Mel, rATG | — | — | Reject+134 | +540 | |
| 18 | IVS8; c.777 + 1G>A | MUD | Treo, Flu, Mel, rATG | — | — | CMV-pneum | — | Death+96 |
| Study group | ||||||||
| 19 | Ex10;с.1201_1205dupССАСС, p.P403HfsTer44 | Haplo | Plerix, G-CSF, Treo, Flu,Mel,rATG | II gr (s) | — | Skin vasc | — | +489 |
| 20 | IVS8; c.777 + 1G>A | MUD | Plerix, G-CSF, Treo, Flu,Mel,rATG | — | — | — | +470 | |
| 21* | Ex11; c.1442_1445dupTCCA, p.S483PfsTer13 | MUD | Plerix, G-CSF, Treo, Flu,Mel,rATG | — | — | — | +498 | |
| 22 | Ex1; с.100С>T, p.R34Ter | Haplo | Plerix, G-CSF, Treo, Flu,Mel,rATG | — | — | — | +400 | |
| 23 | IVS6; c.560-1G>A | Haplo | Plerix, G-CSF, Treo, Flu,Mel,rATG | — | — | Toxo enceph | — | +407 |
| 24 | Ex12; с.1468С>T, p.Q490Ter | Haplo | Plerix, G-CSF, Treo, Flu,Mel,rATG | — | — | — | +370 | |
| 25 | Ex7; c.631C>T, p.R211Ter | Haplo | Plerix, G-CSF, Treo, Flu,Mel,rATG | II gr (s) | lim | Skin vasc | — | +357 |
| 26 | Ex11; с.1453G>A, p.D485N | Haplo | Plerix, G-CSF, Treo, Flu,Mel,rATG | — | — | — | +330 | |
| 171 | Ex4; c.385G>C, p.A129P | MUD | Plerix, G-CSF, Treo, Flu,Mel,rATG | — | — | — | +322 | |
| 27 | Ex2; c.223G>A, p.V75M | Haplo | Plerix, G-CSF, Treo, Flu,Mel,rATG | — | — | CMV-ret | — | +293 |
| 28 | Ex4; с.397G>A, p.E133K | Haplo | Plerix, G-CSF, Treo, Flu,Mel,rATG | — | — | — | +266 | |
| 29 | Ex10; с.961С>T, р.R321Ter | Haplo | Plerix, G-CSF, Treo, Flu,Mel,rATG | — | — | CMV-ret | — | +217 |
| 30 | Ex10;с.1271dupG,p.L425PfsTer70 | MUD | Plerix, G-CSF, Treo, Flu,Mel,rATG | — | — | CMV-ret | — | +176 |
| 161 | Ex3; с.294delA, p.E98DfsTer29 | MUD | Plerix, G-CSF, Treo, Flu,Mel,rATG | — | — | — | +181 | |
| 31 | IVS 6; с.559 + 5G>A | MUD | Plerix, G-CSF, Treo, Flu,Mel,rATG | — | — | — | +92 | |
| 32 | Ex9;c.929_931+9del(AGGgtgagaccc) | Haplo | Plerix, G-CSF, Treo, Flu,Mel,rATG | — | — | Gram-negative sepsis | — | Death+46 |
*Second HSCT after rejection; first HSCT with post-transplantation Cy (+3, +4).
1 Second HSCT after rejection; first HSCT with TCRαβ+/CD19+ graft depletion.
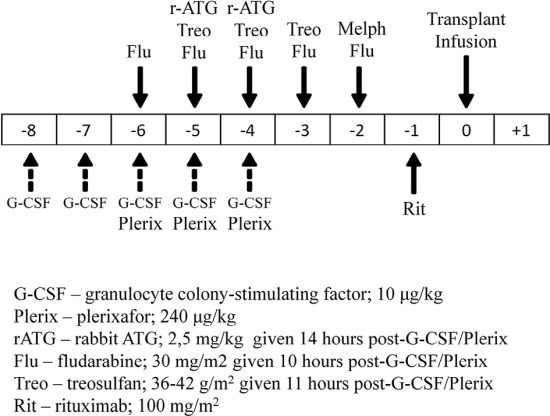
RESULTS
There were no significant differences in initial characteristics, donor types, and transplant compositions between the historical controls and the study group (Table 2). The median follow-up periodwas 16.5 months (range, 3.0 to 60.7 months). The median number of CD34+ cells in the graft was 10.9 × 106/kg (range, 7.6 to 21.3 × 106/kg), whereas that of TCRαβ+cells was 15.5 × 103/kg (range, 2 to 166 × 103/kg).
Early toxicity, such as treosulfan-related skin rash and mucositis, was limited to a few observations and was mild in all cases. In 2 patients in the study group with panniculitis before undergoing HSCT, symptoms of the localized skin inflammation were observed after G-CSF/plerixafor administration. In 1 of these patients, vasculitis was verified by skin biopsy (Figure 2). The skin symptoms in both patients completely resolved within a few days after the G-CSF/plerixafor therapy.
In all patients, neutrophil engraftment occurred during the interval from days +10 to +28 after transplantation (median, day +14), and platelet engraftment occurred between days +7 to +18 (median, day +12). Both neutrophil and the platelet engraftment occurred significantly earlier after G-CSF/ plerixafor administration in the conditioning regimen. The median day of neutrophil engraftmentwas day 12 (range, days 11 to 14) in the study group, compared with day 17 (range, days 16 to 19.75) in the control group (P < .001). The median day of platelet engraftment in the study group was day 10 (range, days 9 to 12) in the study group and day 13 (range, days 13 to 14) in the control group (P = .003) (Figure3).
Table 2
Initial Patient Characteristics, Donor Types, and Transplant Compositions
| Age,yr, median (range) | Ochs Scoring System, median (range) | Transplant Composition, cells/kg; median (range) | Donor type, n | ||||
| TCRαβ+×103 | NC×108 | CD34+×106 | MUD | Haplo | |||
| Historical group | 2 (1-13) | 4 (1-5) | 13 (3.4 -166) | 8.8 (5.7-22.2) | 11.0 (8.9-21.3) | 12 | 6 |
| Study group | 2 (1-12) | 4.5 (3-5) | 20.5 (2-92) | 7.4 (3.7-17.9) | 10.9 (7.6-14.3) | 6 | 10 |
| P value | .63* | .36* | .28* | .08* | .42* | .17† | .17† |
† Fisher’s exact test.
Figure 2. Leukocytoclastic vasculitis after G-CSF/plerixafor administration in a patient with WAS. (A) Red and painful skin lesions on legs. (B) Histological specimen of skin biopsy. (a) Low-power view of leukocytoclastic vasculitis the pattern of a busy dermis with a superficial and mid perivascular inflammatory pattern. (b and c) There are predominantly neutrophils in a perivascular and interstitial pattern in addition to those undergoing extravasation from the vessels. (d) Leukocytoclasis (neutrophil degeneration) forming nuclear dust is seen in addition to extravasated erythrocytes.
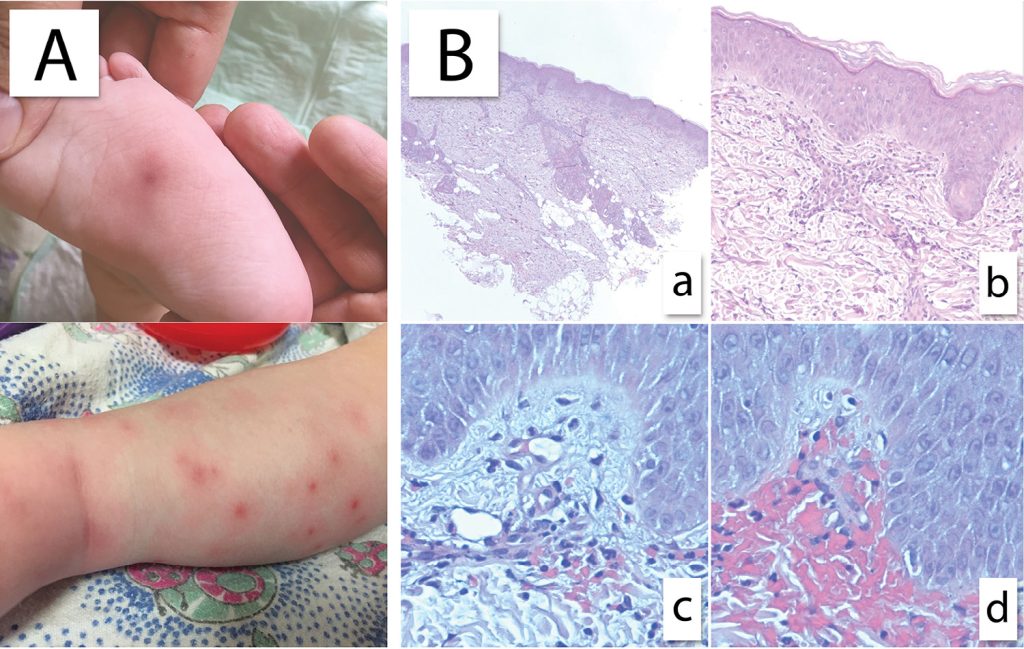
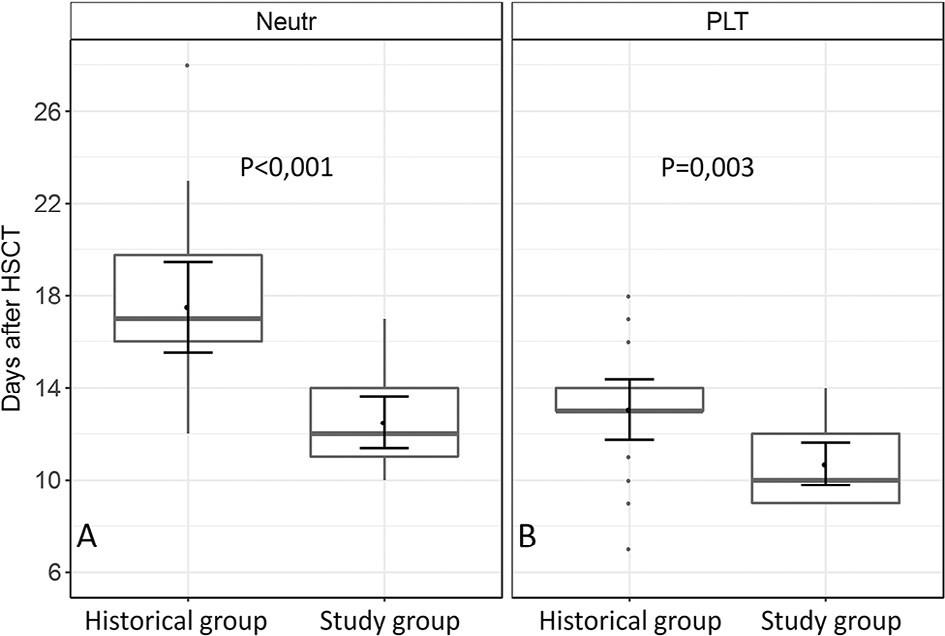
Figure 3. Boxplots for time of neutrophil (A) and platelet (B) engraftment in the historical and study groups.
Infectious Complications
Viral infections were the most common problem after HSCT in both groups. CMV viremia was observed after 19 of the 34 HSCTs (cumulative incidence, 57.0%; 95% CI, 42.3% to 76.8%), and CMV disease occurred after 10 of the 34 HSCTs (29.4%). Nine patients had CMV retinitis, 1 patient had encephalitis with retinitis, and 1 patient had severe CMV pneumonia. One patient developed ADV hepatitis. No patients had EBV-PTLD.
One patient had severe systemic toxoplasmosis (with brain, ocular, lung, and skin involvement), which resolved completely with specific therapy. Another patient with full donor chimerism at day +40 developed lethal Stenotrophomonas maltophilia sepsis.
GVHD
Of the patients withWAS who underwent HSCT, 9 developed grade I/II acute GVHD (cumulative probability, 26.8%; 95% CI, 15.3% to 46.9%). Importantly, no patients had grade III/IV acute GVHD.
All affected patients had only mild GVHD manifestations (3 with skin and intestinal involvement, and 6 with skin involvement only) and responded well to steroid therapy. The cumulative incidence of grade I/II acute GVHD did not differ significantly between patients who underwent HSCT from haploidentical donors (31.1%; 95% CI, 15.1% to 64.6%) and those who underwent HSCT from MUDs (23.0%; 95% CI, 9.7% to 54.4%) (P = .602).
Acute GVHD was observed in 2 of 16 patients in the study group (12.5%; 95% CI, 3.4% to 45.7%) and in 7 of 18 patients in the historical control group (40.2%; 95% CI, 22.6% to 71.5%) (P = .087). One patient developed limited chronic skin GVHD, with good response to low-dose steroids and mycophenolate mofetil.
Chimerism and Graft Failure in the Historical Control
Group
In the historical control group, mixed chimerism in whole blood was observed in 17% of patients at day +30 and in 50% at day +360. Interestingly, mixed chimerism in the CD3+ lineage was found in 55.5% of patients at day +30 and in only 33.3% at day +360 after HSCT (Figure 5a and c).
Mixed chimerism was followed by graft rejection in 5 of 18 patients (27.7%) at 1.8 to 6.9 months after HSCT (median time of graft rejection, 2.7 months). The cumulative incidence of graft rejection was 30.3% (95% CI, 14.5% to 63,2%). Surprisingly, 2 of 6 patients (33.3%) who received the conditioning regimen with the addition of melphalan (Table 1; patients 12, 13, 14, 16, 17, and 19) also rejected the grafts. Two patients with mixed chimerism (at 38 and 39 months after HSCT) from 8 and 25 months after transplantation had severe thrombocytopenia and received romiplostim therapy, with good response (Table 1; patients 7 and 8). Both patients had predominantly recipient mixed chimerism in whole blood but full donor and predominantly donor mixed chimerism in the CD3+ lineage. In all patients who experienced graft rejection or severe thrombocytopenia, full donor or predominantly donor mixed chimerism in the CD3+ lineage but low levels of donor chimerism in whole blood was observed (Figure 4). Therefore, althoughmyeloid chimerism was not assessed directly, it can be indirectly concluded that all cases of severe graft dysfunction were associated with poor myeloid chimerism. The cumulative incidence of graft rejection and severe thrombocytopenia in the historical control group was 42.6% (95% CI, 24.2% to 75.0%).
Chimerism and Graft Failure in the Study Group
All patients in the study group had full donor chimerism in both whole blood and the CD3+ compartment at followup from 3 to 17 months after HSCT ((Figure 5b and d). In contrast to the group of historical control, there were no cases of rejection and severe thrombocytopenia after HSCT with G-CSF and plerixafor in the conditioning regimen. Thus, there was a statistically significant difference in the incidence of rejection and severe thrombocytopenia between the study group and the historical group (P = .006) (Figure 6).
OS and EFS
Three patients died of infectious complications; thus, the cumulative probability of TRM in our studywas only 8.9% (95% CI, .3% to 26%). In the historical control group, 1 patient died from CMV pneumonia (on day +96) and another died from ADV hepatitis (on day +37). In the study group, 1 patient died from S. maltophilia sepsis on day +46. All deceased patients had engraftment and full donor chimerism at the time of death. There was no significant difference in OS between the study group and the historical control group (93.8%; 95% CI, 81.9% to 100% versus 88.9%; 95% CI, 74.4% to 100%; P = .638) (Figure 7A).
Moreover, in all of theWAS patients who underwent HSCT, there was no significant difference in OS between those with MUDs and haploidentical donors (88.5%; 95% CI, 73.6% to 100% versus 93.8%; 95% CI, 81.9% to 100%; P = .612) (Figure 7B).
In the historical control group, no difference in EFS was found when melphalan was added as an additional alkylating agent to the conditioning regimen to decrease graft rejection (P = .716) (Figure S1).
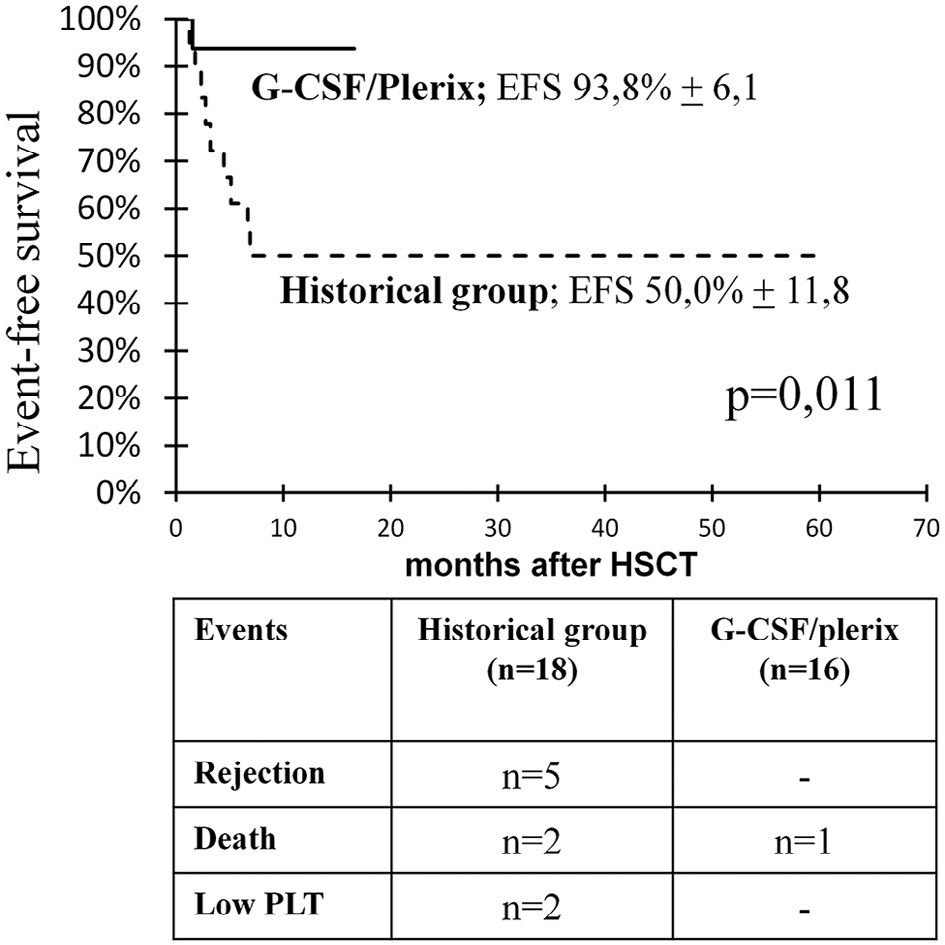
The EFS was 93.8% (95% CI, 81.9% to 100%) in the study group patients who received G-CSF/plerixafor containing conditioning, compared with 50% (95% CI, 26.9% to 73.1%; P = .011) in the control group patients without G-CSF/plerixafor in the conditioning regimen (Figure 8). In the latter group, 5 patients experienced graft rejection, 2 died, and 2 had thrombocytopenia.

Figure 4. Donor cell chimerism in whole blood (A) and the CD3+ lymphocyte compartment (B) after HSCT in 7 patients with severe graft dysfunction (graft rejection, n = 5; severe thrombocytopenia, n = 2).
DISCUSSION
HSCT with TCRαβ+/CD19+ depletion is a fairly new modality for graft manipulation that is becoming increasingly popular among transplantation centers. Since 2012, our center has been using TCRαβ+/CD19+ depletion for HSCT from MUD and haploidentical donors in patients with PIDs. Here we have described our experience with this approach in patients with WAS.
In our cohort, one of the serious complications after HSCT was viral infection; this finding corresponds to the data in previous reports on HSCT with TCRαβ+/CD19+ depletion in patients with PIDs [4,23]. The majority of patients with CMV disease had CMV retinitis, previously reported by Hiwarkar et al. [24] as the most common location of CMV disease in patients with PIDs.
Mixed chimerism is another significant problem after HSCT forWAS. A common approach to preventing this complication is to increase the intensity of preparative chemotherapy regimens, which eradicates the recipient’s residual hematopoietic stem cell pool. However, the escalation of myeloablative chemotherapy regimens increases the frequency and severity of life-threatening complications, leading to increased TRM and morbidity with impaired quality of life. For this reason, despite 50 years of experience with HSCT for WAS, the problem of graft failure has not been solved [13,14].
In this study, 7 of 18 patients (39%) in the historical control group (without G-CSF/plerixafor) developed graft failure (5 with rejection and 2 with thrombocytopenia). Interestingly, in this group, there was no difference in the incidence of graft failure in the patients who received melphalan as a second alkylator.
In May 2016, we began a prospective trial to evaluate the efficacy of G-CSF and plerixafor as part of the conditioning regimen for WAS. For many years, the combination of plerixafor with G-CSF was used mainly in autologous HSCT for hematopoietic stem cell mobilization [25,26]., but it was discovered that the mechanism of action of plerixafor and G-CSF could be applied to other clinical situations as well. The successful use of plerixafor and G-CSF administration for leukemic cell mobilization followed by chemotherapy in the conditioning regimen before allogeneic HSCT in patients with acutemyelogenous leukemiawas reported by Кonopleva et al. [18]. We used this combination in our study to enhance the myeloablative effect by blocking stem cell homing and increasing the sensitivity of stem cells to chemotherapy.
Interestingly, neutrophil and platelet engraftment occurred significantly earlier in the patients who received G-CSF/plerixafor-containing conditioning compared with the control group. This might be due to the more efficient engraftment of stem cells to vacant bone marrow niches after G-CSF/plerixafor administration.
Despite the shorter follow-up in the study group and the lack of late chimerism data compared with the historical controls, the results of this protocol appear promising, considering that no patients developed mixed chimerism at 3 to 17 months of follow-up (Figure 5b and d) despite the prevalence of haploidentical donors in this group. There were no cases of graft failure or autoimmune complications after G-CSF/plerixafor-containing conditioning. Thus, the EFS was significantly higher in the patients who received G-CSF/plerixafor-containing conditioning (93.8%) compared with those in the historical control group. The sole event in this group was a death from Gram-negative sepsis in 1 patient with full donor chimerism (Figure 8).
In 2 patients, G-CSF/plerixafor therapy was followed by transient leukoclastic skin vasculitis, with complete resolution of symptoms a few days after G-CSF/plerixafor administration. The vasculitis symptoms were mild and did not lead to any significant consequences. Both patients had severe panniculitis before HSCT. This effect is most likely related to the exacerbation of the autoimmune complications observed in patients with WAS before HSCT, because there was no reference to such side effects in patients with malignant disorders or in healthy donors receiving G-CSF/plerixafor before cytopheresis [18,19,27].
The incidence of acute GVHD was low in all of the patients with WAS; all cases were limited to grade I/II and demonstrated good response to the first line of therapy. There were no cases of grade III/IV acute GVHD and only 1 case of limited chronic GVHD. The low incidences of severe acute and chronic GVHD in our study were comparable with those in reports on HSCT with TCRαβ+/CD19+ depletion in PID by Italian and British groups and with the results of our previous study [3,4,12]. Importantly, there was a trend toward a lower incidence of acute GVHD in patients receiving G-CSF/plerixaforcontaining conditioning (P= .087). Similarly, Konopleva et al. [18] reported a decreased incidence of acute GVHD after G-CSF/plerixafor administration.
Owing to the low incidence and severity of GVHD after HSCT with TCRαβ+/CD19+ depletion, this complication did not influence survival. Of note, there were no statistically significant differences in the probabilities of GVHD and OS between patients who underwent HSCT from haploidentical donors and those who underwent HSCT from MUDs. Therefore, TCRαβ+/CD19+ graft depletion may be recommended for HSCT from haploidentical donors in a wider spectrum of clinical situations.
TCRαβ+/CD19+ depletion is an appropriate approach for HSCT from alternative donors. To our knowledge, this is the first report to suggest equal outcomes in patients withWAS after MUD and haploidentical HSCT performed with identical transplantation protocols. However, the high incidence of mixed chimerism in patients with WAS after TCRαβ+/CD19+ graft depletion in the historical group compromised the success of HSCT. The addition of the noncytotoxic agents G-CSF and plerixafor to the preparative regimen before HSCT appears to solve the problem of graft failure after HSCT, with no additional risks of toxic complications and associated morbidity. The present study supports the efficacy of this approach. The use of G-CSF/plerixafor in combination with less-intensive chemotherapy regimens merits further investigation, particularly for nonmalignant conditions in which full donor chimerism is preferable.
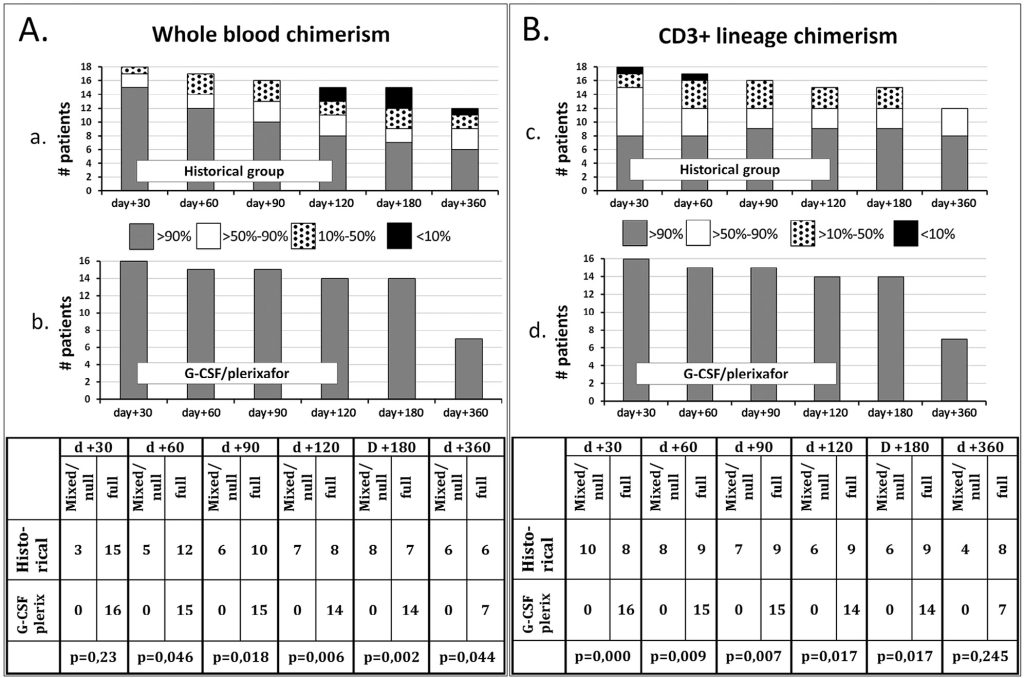
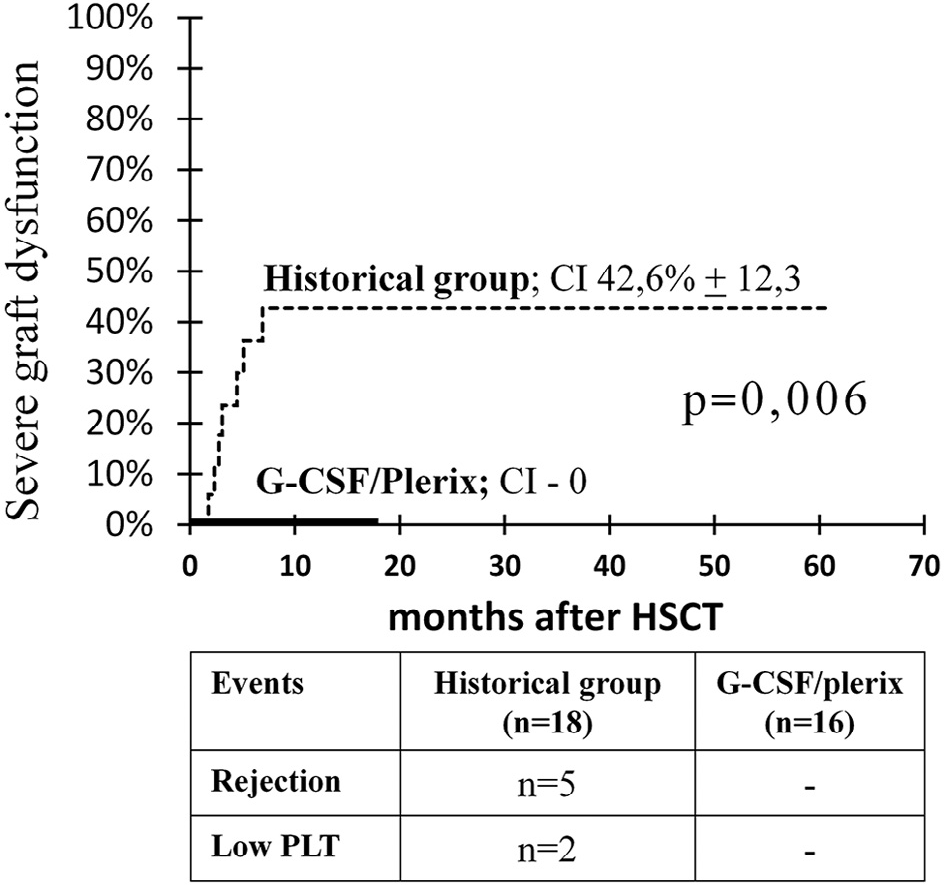
Figure 6. Cumulative incidence of severe graft dysfunction (rejection and severe thrombocytopenia) in the study group compared with the historical group.
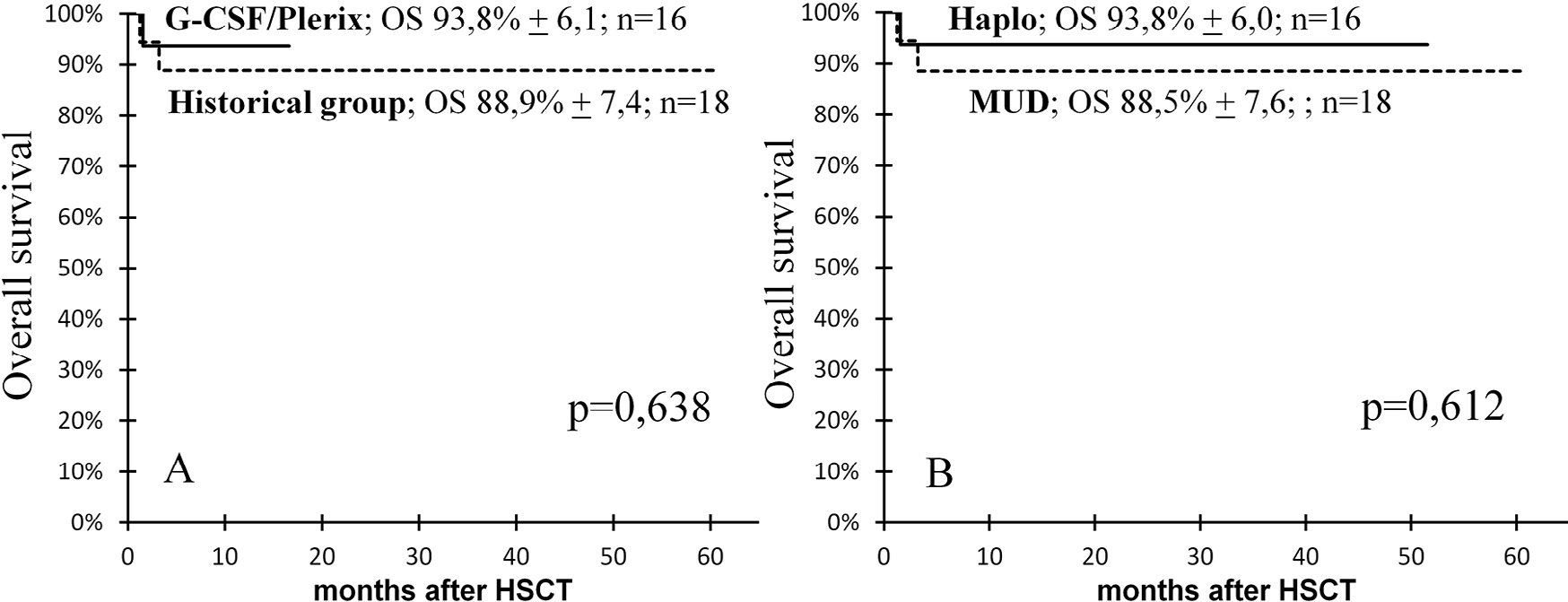
Figure 8. EFS in the patients after G-CSF/plerixafor administration in the conditioning regimen and in the historical control group.
ACKNOWLEDGMENTS
The authors thank Marina Persiantseva for international registry coordination; Elena Kurnikova, Yakov Muzalevskiy, and Alexei Kazachenok for graft processing; Elena Boyakova and Dmitry Pershin for lymphocyte phenotyping; Varvara Brilliantova for chimerism analysis; and Dr. Andrew Gennery (University of Newcastle upon Tyne, UK) for a review and helpful suggestions.
Financial disclosure: The authors have nothing to disclose.
Conflict of interest statement: There are no conflicts of interest to report.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found online at doi:10.1016/j.bbmt.2018.03.006.
REFERENCES
1. Handgretinger R. Negative depletion of CD3(+) and TcRaβ(+) T cells. Curr Opin Hematol. 2012;19:434-439. 2. Oevermann L, Lang P, Feuchtinger T, et al. Immune reconstitution and strategies for rebuilding the immune system after haploidentical stem cell transplantation. Ann N Y Acad Sci. 2012;1266:161-170. 3. Bertaina A, Merli P, Rutella S, et al. HLA-haploidentical stem cell transplantation after removal of αβ+ T and B cells in children with nonmalignant disorders. Blood. 2014;124:822-826. 4. Shah RM, Elfeky R, Nademi Z, et al. T-cell receptor αβ+ and CD19+ cell-depleted haploidentical and mismatched hematopoietic stem cell transplantation in primary immune deficiency [e-pub ahead of print]. J Allergy Clin Immunol. 2017. doi:10.1016/j.jaci.2017.07.008. Accessed July 10, 2017. 5. Kharya G, Nademi Z, Leahy TR, et al. Haploidentical T-cell alpha beta receptor and CD19-depleted stem cell transplant for Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2014;134:1199 1201. 6. Ghosh S, Schuster FR, Adams O, et al. Haploidentical stem cell transplantation in DOCK8 deficiency—successful control of pre-existing severe viremia with a TCRaß/CD19-depleted graft and antiviral treatment. Clin Immunol. 2014;152:111-114. 7. Pai SY, Notarangelo LD. Hematopoietic cell transplantation for Wiskott- Aldrich syndrome: advances in biology and future directions for treatment. Immunol Allergy Clin North Am. 2010;30:179-194. 8. Dupuis-Girod S, Medioni J, Haddad E, et al. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single center cohort of 55 patients. Pediatrics. 2003;111(5 Pt 1):e622-e627. 9. Imai K, Morio T, Zhu Y, et al. Clinical course of patients withWASP gene mutations. Blood. 2004;103:456-464. 10. Schurman SH, Candotti F. Autoimmunity in Wiskott-Aldrich syndrome. Curr Opin Rheumatol. 2003;15:446-453. 11. Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the Wiskott-Aldrich syndrome. J Pediatr. 1994;125(6 Pt 1):876-885. 12. Balashov D, Shcherbina A, Maschan M, et al. Single-center experience of unrelated and haploidentical stem cell transplantation with TCRαβ and CD19 depletion in children with primary immunodeficiency syndromes. Biol Blood Marrow Transplant. 2015;21:1955-1962. 13. Moratto D, Giliani S, Bonfim C, et al. Long-term outcome and lineagespecific chimerism in 194 patients with Wiskott-Aldrich syndrome treated by hematopoietic cell transplantation in the period 1980-2009: an international collaborative study. Blood. 2011;118:1675-1684. 14. Ochs HD, Filipovich AH, Veys P, Cowan MJ, Kapoor N. Wiskott-Aldrich syndrome: diagnosis, clinical and laboratory manifestations, and treatment. Biol Blood Marrow Transplant. 2009;15(1 Suppl):84-90. 15. Ozsahin H, Cavazzana-Calvo M, Notarangelo LD, et al. Long-term outcome following hematopoietic stem-cell transplantation in Wiskott-Aldrich syndrome: collaborative study of the European Society for Immunodeficiencies and European Group for Blood and Marrow Transplantation. Blood. 2008;111:439-445.
16. Uy GL, Rettig MP, Motabi IH, et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 2012;119:3917-3924. 17. Chen J, Larochelle A, Fricker S, Bridger G, Dunbar CE, Abkowitz JL. Mobilization as a preparative regimen for hematopoietic stem cell transplantation. Blood. 2006;107:3764-3771. 18. Konopleva M, Benton CB, Thall PF, et al. Leukemia cell mobilization with G-CSF plus plerixafor during busulfan fludarabine conditioning for allogeneic stem cell transplantation. Bone Marrow Transplant. 2015;50:939-946. 19. Dvorak CC, Horn BN, Puck JM, et al. A trial of plerixafor adjunctive therapy in allogeneic hematopoietic cell transplantation with minimal conditioning for severe combined immunodeficiency. Pediatr Transplant. 2014;18:602-608. 20. ESID Registry. Working definitions for clinical diagnosis of PID. Available at https://esid.org/content/download/15783/432111/file/ESID Registry_ClinicalCriter.pdf. Accessed April 25, 2017. 21. Alizadeh M, Bernard M, Danic B, et al. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood. 2002;99:4618- 4625. 22. Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825-828. 23. Laberko A, Bogoyavlenskaya A, Shelikhova L, et al. Risk factors for and the clinical impact of cytomegalovirus and Epstein-Barr virus infections in pediatric recipients of TCR-α/β- and CD19-depleted grafts. Biol Blood Marrow Transplant. 2017;23:483-490. 24. Hiwarkar P, Gajdosova E, Qasim W, et al. Frequent occurrence of cytomegalovirus retinitis during immune reconstitution warrants regular ophthalmic screening in high-risk pediatric allogeneic hematopoietic stem cell transplant recipients. Clin Infect Dis. 2014;58: 1700-1706. 25. Fowler CJ, Dunn A, Hayes-Lattin B, et al. Rescue from failed growth factor and/or chemotherapy HSC mobilization with G-CSF and plerixafor (AMD3100): an institutional experience. Bone Marrow Transplant. 2009;43:909-917. 26. Sinha S, Gastineau D, Micallef I, et al. Predicting PBSC harvest failure using circulating CD34 levels: developing target-based cutoff points for early intervention. Bone Marrow Transplant. 2011;46:943-949. 27. Maschan AA, Balashov DN, Kurnikova EE, et al. Efficacy of plerixafor in children with malignant tumors failing to mobilize a sufficient number of hematopoietic progenitors with G-CSF. Bone Marrow Transplant. 2015;50:1089-1091.
